Ozone Layer – Earth’s Protective Shield
Introduction to Ozone Layer
The ozone layer is a region of Earth’s stratosphere that absorbs most of the Sun’s ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth’s atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers above Earth, although its thickness varies seasonally and geographically.
The ozone layer was discovered in 1913 by French physicists Charles Fabry and Henri Buisson. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature in the range of 5,500–6,000 K (5,230–5,730 °C), except that there was no radiation below a wavelength of about 310 nm at the ultraviolet end of the spectrum. It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually the spectrum of the missing radiation was matched to only one known chemical, ozone. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The “Dobson unit”, a convenient measure of the amount of ozone overhead, is named in his honor.
Importance of Ozone Layer
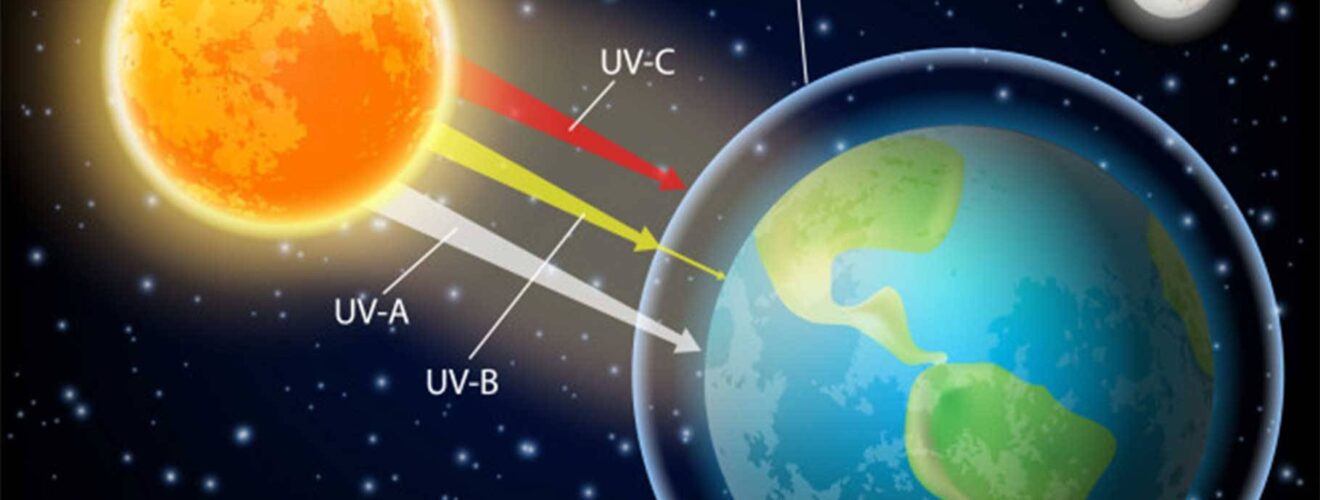
UV Radiation Absorption
Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation coming from the Sun. Extremely short or vacuum UV (10–100 nm) is screened out by nitrogen. UV radiation capable of penetrating nitrogen is divided into three categories, based on its wavelength; these are referred to as UV-A (400–315 nm), UV-B (315–280 nm), and UV-C (280–100 nm).
Ecosystem Protection
UV radiation can have detrimental effects on marine ecosystems, terrestrial plants, and animals. The ozone layer serves as a crucial barrier, preventing the harmful impacts of excessive UV exposure on biodiversity.
Depletion of Ozone Layer

The ozone layer can be depleted by free radical catalysts, including nitric oxide (NO), nitrous oxide (N2O), hydroxyl (OH), atomic chlorine (Cl), and atomic bromine (Br). While there are natural sources for all of these species, the concentrations of chlorine and bromine increased markedly in recent decades because of the release of large quantities of man-made organohalogen compounds, especially chlorofluorocarbons (CFCs) and bromofluorocarbons. These highly stable compounds are capable of surviving the rise to the stratosphere, where Cl and Br radicals are liberated by the action of ultraviolet light. Each radical is then free to initiate and catalyze a chain reaction capable of breaking down over 100,000 ozone molecules. By 2009, nitrous oxide was the largest ozone-depleting substance (ODS) emitted through human activities.
Effects of Ozone Depletion
Increased Skin Cancer Risk
With reduced ozone protection, more UV radiation reaches the Earth’s surface, increasing the risk of skin cancers and other health issues in humans.
Environmental Impact
Ozone depletion can harm aquatic ecosystems, disrupt food chains, and affect the growth and development of various plant species.
Implication of Astronomy
As ozone in the atmosphere prevents most energetic ultraviolet radiation reaching the surface of the Earth, astronomical data in these wavelengths have to be gathered from satellites orbiting above the atmosphere and ozone layer. Most of the light from young hot stars is in the ultraviolet and so study of these wavelengths is important for studying the origins of galaxies. The Galaxy Evolution Explorer, GALEX, is an orbiting ultraviolet space telescope launched on April 28, 2003, which operated until early 2012.
Lesson Learned
The ozone layer stands as a testament to the planet’s ability to heal when concerted efforts are made. As we continue to address the challenges posed by ozone layer depletion, it is crucial to remain vigilant, enforce environmental policies, and embrace sustainable practices to ensure the long-term health of this vital protective shield.








