New Cancer Treatment With Proton
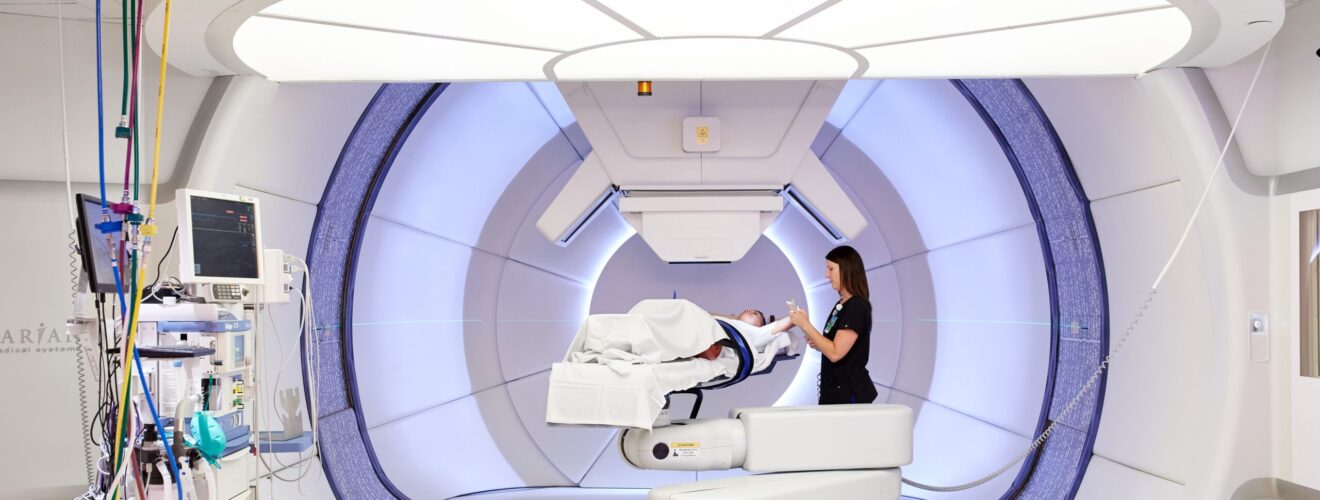
We have talked a lot about particle accelerators that look for new physics. But sometimes you can teach new tricks to old physics. A great example of this is flash proton therapy for cancer treatment, that’s just out of the laboratory and now being tested on patients in the first trials.
Use of proton therapy
First things first, what does particle physics have to do with cancer? Well, one of the most common ways to treat cancer is radiation therapy with X-rays. So that’s particles of light, the photons, with very high energy each. You can use these highly energetic photons to kill off cancer cells. The difficulty really isn’t killing the cancer cells; it’s killing the cancer cells without killing the patient. But the problem with using X-rays is that you can’t shoot them at tumours inside the body without also burning some of the tissue on the way to the tumour and behind it. This is because the X-ray beam deposits its energy gradually in the tissue, sometimes too early and sometimes too late. One can somewhat alleviate this issue by rotating the beam around, so that it overlaps most in the cancer region, but the problem that energy gets deposited where it shouldn’t go is unavoidable. This can damage healthy cells and brings the risk of secondary cancers from the radiation treatment That’s if you use X-rays.
But you can use beams of other particles instead, and this is where particle physics enters the chat. Because with heavier particles you get a more targeted energy delivery. It’s a case where a graph says more than a thousand words so let me draw one for you. On the vertical axis you have a measure for the energy that’s deposited into the tissue, that’s called the dose. On the horizontal axis you have the length of the path in the tissue. For X-rays the curve looks somewhat like this. It does have a peak where the beam deposits most of the energy, but it’ll dump significant amounts over a pretty big stretch of distance. That’s the problem which damages healthy tissue. But if you instead take a beam of protons, the energy deposit in the tissue looks like this. A proton, just to remind you, is the nucleus of a hydrogen atom and is what the Large Hadron Collider collides.
Bragg Peak
You see that a beam of protons is far less likely to interact with tissue on short distances, then the energy deposit peaks rather suddenly, and tapers off quickly. The peak in this curve is called the “Bragg peak”. It’s named after the father-and-son duo Sir William Henry Bragg and Sir William Lawrence Bragg, who were British physicists. They were awarded the Nobel Prize in Physics in 1915 for work related to this peak. I guess that was the Bragg peak of their career. What’s going on with this Bragg peak is that the protons slow down as they lose energy whereas light doesn’t. So you see the heavier particles bump into one atom in the tissue and lose a little energy there. Then the distance they cover until they hit the next atom is shorter because they’re slower, and then it gets even shorter, until they’ve dumped all their energy. What happens with the protons in the end? They usually get absorbed by some nuclei. Isn’t that kind of bad? Yes, it’s kinda bad but remember you’re doing it to destroy the tumour cells, it’s supposed to be bad, just that it should be bad in the right place. The relevant thing is that what’s left behind is mostly benign biological trash that the body can dispose of.
Interestingly, if you take even heavier particles, the Bragg peak gets even more pronounced at a very specific distance. You could use for example nuclei of carbon atoms. You see how cool this is, you can basically use these beams to shoot at a tumour inside the body without roasting the tissue on the way there. The idea isn’t new. For example, at the GSI in Germany they ran a trial for using beams of carbon ions to treat cancer already in 1997. Mostly that’s brain tumours where you want to make extra sure you don’t damage surrounding tissue. This sounds great, so why isn’t this done more often? The issue is that the bigger the particle, the bigger the accelerator you need to get them up to speed, and the more expensive and difficult the entire procedure becomes.
Why Proton Therapy is still uncommon?
This is why proton therapy is still rather uncommon, and that with heavier ions even less common. But particle accelerators are getting more efficient and smaller and also more affordable and that’s why proton therapy is now being offered in more places. For example, the company Varian Medical Systems offers a proton beam machine for medical purposes at 20 million dollars a pop. At Ohio State University they just recently acquired a machine of the same type and last week they announced that they’ll be testing a new treatment procedure called Flash proton therapy. The idea of flash proton therapy is that rather than irradiating the tumour continuously for a few minutes, the same total dose is delivered packed into pulses lasting only a second or so. So, the flashes. Doctors have known for some time that this seems to work better in that it delivers the damage more precisely to the tumour region. But they don’t really understand why and the treatment method has only been out of the lab for a few years. There is a surprising amount of physics in medical treatments, and personally I find this flash proton procedure one of the best examples.